Harper Highlights: Ovarian Cancer Research at Harper Cancer Research Institute (HCRI)
M. Sharon Stack, PhD., Ann F. Dunne & Elizabeth Riley Director
This year, it is estimated that 22,280 women in the United States will be diagnosed with ovarian cancer and 15,500 will die of the disease. While the 5-year survival rates of women with breast and cervical cancers are now 89% and 70%, respectively, the 5-year survival rate of women with ovarian cancer remains below 50%. These statistics highlight the urgent need for enhanced research to improve early detection, develop novel treatment strategies and combination therapies, and devise methods to more effectively image intra-peritoneal metastatic disease. Researchers at the Harper Cancer Research Institute (HCRI) are investigating many aspects of ovarian cancer diagnosis, progression and metastasis, and treatment.
Investigation of the molecular basis of cellular changes that accompany the onset and early spread of epithelial tumors is the focus of Professor Crislyn D’Souza-Schorey’s research in the Department of Biological Sciences at Notre Dame. Tumor cells shed a specialized population of small membrane-enclosed microvesicles that can be detected in body fluids including blood, urine and ascites. These structures contain protein and nucleic acid cargoes that can be released into the environment at a distant site or transferred to another cell. In collaboration with gynecologic oncologists Drs. Michael Method and Michael Rodriguez at Michiana Hematology Oncology, the D’Sousa-Schorey laboratory is working on a diagnostic platform focused on ovarian cancer. These efforts include the identification, quantification and characterization of ovarian tumor-derived microvesicles. Ongoing studies are aimed at identifying specific protein markers and morphological characteristics of the vesicles as well as developing clinically adaptable methods to consistently isolate these structures from clinical cohorts. The microvesicle platform is advantageous as it ‘isolates’ molecular changes in the tumor and thus increases the sensitivity of identifying critical markers of tumor progression or disease recurrence. Thus microvesicles show promise as robust and informative circulating biomarkers to enable new early detection and disease-staging strategies that could augment current regimens.
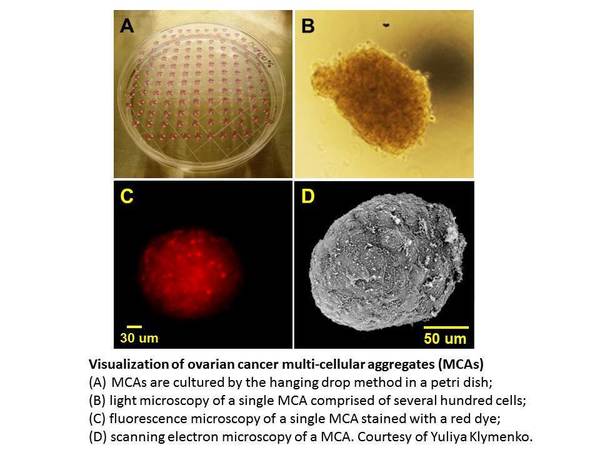
Unlike other solid tumors, hematogenous metastasis of ovarian cancers is uncommon. Instead, an early event in metastatic dissemination is exfoliation of cells from the primary tumor into the peritoneal cavity as individual cells and multi-cellular aggregates. Shed tumor cells interact with mesothelial cells lining the inner surface of the peritoneal cavity, anchor within the sub-mesothelial matrix, and proliferate to establish secondary lesions. The peritoneal cavity thus provides an unique microenvironmental niche comprised of tumor cells, inflammatory components and a host of soluble factors. The primary tumor as well as both suspended and anchored metastatic cells, often maintain direct contact with ascites, providing a mechanism for dynamic regulation of the tumor microenvironment. Regulation of tumor cell dissemination within this unique niche is a major focus of research in the laboratory of M. Sharon Stack, Professor of Chemistry and Biochemistry at the University of Notre Dame. Using primary and established human ovarian tumor cells, the Stack laboratory is investigating the role of reversible changes in the expression and function of various adhesion molecules in ovarian cancer metastasis. More recent studies have begun to evaluate the contribution of the “host” tissues to ovarian cancer metastatic success. For example, as the average age at which ovarian cancer is diagnosed is 63, research is addressing the hypothesis that ageing alters the mesothelial micro-architecture and therefore the ‘receptivity’ of the peritoneal mesothelium and omentum to metastatic implantation by tumor cells. Additional studies are evaluating the potential contribution of obesity to ovarian cancer metastatic success. Initial results suggest that tumor cells adhere more avidly to peritoneal tissues of mice fed on a high fat “Western” diet relative to control animals. Another factor relatively unique to the ovarian tumor microenvironment is the accumulation of peritoneal ascites, often in volumes >1 liter, that occurs in the majority of women with late stage ovarian cancer. The effect of ascites-induced changes in intra-peritoneal fluid pressure on both tumor cell metastatic potential and host cell receptivity is the focus of an ongoing collaboration between the Stack laboratory and Diane Wagner, Associate Professional Specialist in the Department of Aerospace and Mechanical Engineering at the University of Notre Dame. As the vast majority of women with ovarian cancer are diagnosed after metastatic dissemination has already occurred, the long term goal of these studies is to gain a molecular-level understanding of metastasis to enable the development of novel therapies to inhibit intra-peritoneal spread.
The current standard of care for ovarian cancer is surgery followed by chemotherapy, usually with a platinum compound and frequently paclitaxel. Patients initially respond well to chemotherapy, yet most relapse after the initial treatment, and very few options are available to effectively treat platinum-resistant recurrent disease. Many factors, including altered cell survival, DNA repair, drug efflux and the presence of cancer stem cell (CSC) populations may contribute to chemoresistance. Research in the laboratory of Karen Cowden-Dahl, Assistant Professor of Biochemistry and Molecular Biology at the Indiana University School of Medicine South Bend (IUSMSB) is focused on the role of ARID3B, a transcription factor that is overexpressed in high grade serous ovarian cancer. Although the exact role of ARID3B in ovarian cancer is currently unclear, it has been shown to transform cells and promote tumor growth and migration in other model systems. Recent data from the Cowden-Dahl laboratory suggest that ARID3B promotes the formation and/or maintenance of ovarian cancer stem cells via transcriptional regulation of a CSC-specific genetic signature. Uncovering the potential link between the ARID3B-regulated acquisition of CSC markers and the chemoresistant phenotype is a major focus of the Cowden-Dahl laboratory. These studies may unveil a genetic signature that can be used in the future to target novel therapies to chemoresistant populations of cells.
Several highly potent chemotherapeutic agents fail in clinical trials as a result of their systemic toxicity. Nanotechnology is a potentially paradigm-changing opportunity for drug delivery to circumvent this issue. Assistant Professor Basar Bilgicer, in the Department of Chemical and Biomolecular Engineering at the University of Notre Dame, is developing biomolecules and nanoparticles to carry chemotherapeutic drugs that are released selectively when the particles attach to the cancerous cells. Using combined expertise in bioengineering, biophysical chemistry, synthetic chemistry, and targeted drug delivery, the Bilgicer laboratory is focused on the design of multivalent molecules in an effort to redesign drugs used in chemotherapy for enhanced selectivity. An important feature of nanoparticles is that they present particularly attractive scaffolds for the display of multiple functional groups on their surfaces. Receptor-directed targeting of nanoparticles results in selective binding to tumor cells and enhanced intracellular delivery of the drug, adding another level of sophistication to traditional drug delivery systems. The design of nanoparticulate formulations of chemotherapeutics may not only improve their therapeutic indices, but also allow for multi-drug loading and targeting formulations for increased tumor accumulation and improved efficacy in ovarian cancer.